New study questions earlier conclusions about the kinetics of T cell receptors
T cell receptors are among the most important molecules in the immune system because of their role in recognizing the antigens that signal such threats as viruses and cancer. The receptors must also distinguish these threats from the body's own cells to avoid triggering an unwanted immune system response. Recognition requires direct physical contact between the receptor and the antigen. Researchers attempting to understand this critical mechanism, therefore, have been studying such factors as the affinity for interaction between antigens and T cell receptors, how long those interactions last and how rapidly they occur. Information about these interactions has come mostly from studying receptor molecules removed from the outer membranes of T cells – the location where they normally operate.
Now, a paper scheduled to be published March 31 in the journal Nature questions much of what had been believed about the kinetics of T cell receptors. Based on two techniques that mechanically study receptors as they operate on T cell membranes, the findings could lead to a reevaluation of earlier conclusions.
"We compared parameters that had been measured by using purified T cell receptor molecules to the parameters we measured from T cell receptors on the surfaces of cells, and we found dramatic differences," said Cheng Zhu, a Regents professor in the Coulter Department of Biomedical Engineering at the Georgia Institute of Technology and Emory University. "We don't yet fully understand why the T cell receptor behaves differently when it is located on the surface of a cell compared to when it is purified in solution, but this may be a warning to reconsider earlier conclusions."
The research, done in collaboration between Georgia Tech and Emory University, was sponsored by the National Institutes of Health and the National Multiple Sclerosis Society.
In their studies of two-dimensional receptor-antigen interactions, the researchers found as much as 8,300 times more rapid off-rates than earlier studies. More importantly, they found that the strongest interactions with antigens turned on and off the most quickly – a finding exactly opposite what had been observed in purified T cell receptors.
"The earlier conclusions state that the interaction with the most potent antigens remained stable for a long time to allow many steps of the resulting cascade to occur before dissociation," said Veronika Zarnitsyna, a research scientist in Zhu's laboratory. "But we found that the most potent antigen actually dissociated the fastest. Since mathematical models of T cell discrimination of antigens are based on these earlier conclusions, our findings may cause people to rethink what has been done."
When measured on the cell surfaces, the molecular interactions show an affinity range about 100 times greater and an on-rate range about 1,000 times greater than what had been reported in studies with purified T cell receptors. That is potentially important, Zhu says, because the much broader dynamic ranges found in the new research can now match the range over which the T cells respond to antigens – whereas the narrower ranges of the previous measurements could not.
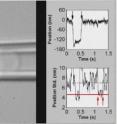
Zhu's research team has been studying two-dimensional molecular interactions for more than a decade, and developed a simple mechanical technique – known as the adhesion frequency assay – for assessing interactions on cell surfaces. On one micropipette, they place a T cell that they want to study. On another micropipette, they put a red blood cell on which an antigen – technically known as a peptide-major histocompatibility complex – has been placed. They then carefully move the two cells together, allowing the antigen and receptor to make contact.
"When the molecules interact, they actually link the two cells together," Zhu explained. "We can see the interaction through the microscope if the two cells remain stuck together when we try to pull them apart."
By measuring the elongation of the cells and the time required to create the binding, the researchers can learn about the interaction. Under computer control, the assays are repeated as many as 100 times to estimate the frequency, or the likelihood, of the interactions.
"We can measure the frequency versus the contact time," said Zhu. "From that information, we can determine the kinetics of the interaction."
The second technique, known as thermal fluctuation assay, detects the interactions from changes in the fluctuations due to a physical anchorage between the two surfaces.
The researchers studied the responses of just one T cell receptor to seven different antigens. Since there are millions of different T cell receptors in the body, the researchers would like to study responses of additional receptors to see if what they found is a general principle.
"Next, we plan to study a single peptide against a panel of T cell receptors to see whether the same principles apply," said Zhu. "We need to see whether or not this is a general principle governing the interaction of T cell receptors."
For the study reported in Nature, the team used T cells provided by Lindsay Edwards, who also measured the functional T cell responses. Edwards is a student of Brian Evavold, a professor in the department of microbiology and immunology at Emory University. In addition to Zhu, Zarnitsyna, Edwards and Evavold, the paper's authors include postdoctoral scholar Baoyu Liu and two former graduate students in Zhu's lab, Jun Huang and Ning Jiang.
The new findings could be important not only for scientists who study the immune system, but also for researchers fighting the war on cancer and companies producing vaccines.
"Everything starts with the T cell receptor, and the interaction has to be a direct physical contact between the T cell and another cell in the body," Zhu noted. "This work provides a new framework for understanding how the T cell receptor function works."
Source: Georgia Institute of Technology Research News
Other sources
- New study questions earlier conclusions about the kinetics of T cell receptorsfrom Biology News NetWed, 31 Mar 2010, 22:49:09 UTC
- New study questions earlier conclusions about the kinetics of T cell receptorsfrom Science DailyWed, 31 Mar 2010, 19:28:52 UTC